Quantitative chemical methods
Goal(s)
Main objective
The concrete has to maintain adequate durability in the assumed environmental conditions with low maintenance expenditure for the designed operational time. During the use period, concrete structures are exposed to many environmental factors causing concrete degradation. From the projecting phase the main influence on the behaviour of concrete in real constructions is the initial composition, especially – type and quantity of cement, water/cement ratio, quality of aggregate, type and quantity of additives. Performance of concrete can be modified by reduction of the water quantity in the mixture or use of cement with mineral additives, limiting the capillary porosity of the grout cement on the structural scale. In global scale this influences directly the penetration depth of aggressive media and level of the diffusion of aggressive ions into the interior of the cement matrix. However, there are many factors influencing the final performance of concrete in various environments and applications.
Concrete subjected to the aggressive ions environment will corrode at different rates depending on the concentration of different salts. Main types of corrosion processes influenced by different salt concentrations are: chloride corrosion, sulphate corrosion and alkali-aggregate reaction corrosion. Chloride ions penetrate very fast into the deepest parts of the cement matrix, therefore the chloride corrosion proceeds quickly into the structure. Presence of the chloride ions lead to the reduction of pH and formation of the compounds which increase their volume and that can result in cracks and other deformations. Main problem arising from the chloride corrosion as well as for other mechanisms of this process is the corrosion of reinforced steel bars. The source of the chloride ions can be assigned to mine waters or sea waters, but most of all from defrosting detergents. The penetration level depends on the cycles of saturation, drying of the surface, freeze thaw.
Sulphate corrosion is one of the most dangerous type of the degradation process in the life cycle of the concrete structures. Sulphate ions presence is directly connected with influence of groundwater, sewage, fertilizers, industrial waste. In case of sulphate corrosion there are two types of propagation – external or internal. External attack occurs when concrete is subjected to the sulphate solutions and ions react with cement matrix forming gypsum or ettringite.This leads to the expansion inside the structure and cracking. Internal attack occurs when in the structure there is cement with high content of gypsum, which leads to excessive content of sulphates from the beginning. The problem also affects concrete subjected to thermal treatment.
Alkali-aggregate reaction corrosion is a destructive process resulting from the reaction of sodium and potassium hydroxides in the pores of the concrete with reactive silica from certain aggregates. Mostly affected types of are those reach in active silica and carbonate aggregates. The resulting sodium-potassium-calcium silica gel expands by absorbing water and the induced pressure causes cracking and damages inside the structure. This mechanism is especially dangerous for concretes used in road construction, for instance pavements or bridge elements. The alkaline reactivity of aggregates is a complex phenomenon, both in terms of the type of alkaline reactions and the variety of mineralogical, chemical and atmospheric factors influencing the progress of alkali-aggregate reaction.
For these purposes several chemical methods are used in order to quantitatively assess the rate of degradation processes due to mechanisms mentioned above, which can be generally divided into groups such as analytical and electrochemical methods. Those will be detailed below.
Description
Functioning mode
- Electroanalytical methods:
- Potentiometric titration - method base on the potential difference between the indicator electrode and the reference electrode after adding portions of titration substance. Selected indicator electrode reacts to changes in the concentration of the analyte or the titration reagent .
- Chloride Diffusion Test/ Ions migration in electrical field – method base on accelerated movement of the chloride ions in concrete sample, induced by electrical field. Test is performed in two chambers with electrodes separated by concrete sample and the concentration changes of chlorides penetrating the cathode are monitored. Samples are usually cut in the form of 50 mm thick discs from 100 mm diameter cylinders and saturated in solution of NaOH. upstream reservoir is filled with NaOH and NaCl solution while the downstream reservoir is filled with NaOH solution only. Power supply is connected to the sample that the negative pole is attached to the upstream cell and the positive pole is attached to the downstream cell. Samples from the upstream and downstream reservoirs are taken periodically and analysed for chloride content .
- Galvanostatic pulse technique - current pulse is applied galvanostatically with the external counter electrode over the concrete surface and the resultant change in potential is detected; A current pulse is imposed on the reinforcement from a counter electrode placed on the concrete surface. A guard ring confines the current to an area A of the reinforcement below the central counter electrode. The applied current is usually in the range of 5 to 400 A and the typical pulse duration is 5 to 10 seconds. The reinforcement is polarized in the anodic direction compared to its free corrosion potential. The resulting change of the electrochemical potential of the reinforcement is recorded as a function of time using a reference electrode (Ag/AgCl). Modern equipment for the galvanostatic pulse technique can be also used in non-destructive testing directly on the tested area .
- Analytical methods:
- Argentometric titration – method allows the quantitative determination of a specific analyte dissolved in a sample based on a chemical reaction between the analyte and a titrant of known concentration. Precipitation of silver compounds is used to determine chloride content in the samples Argentometric titration is usually working in three modes – direct, back compensated or blank compensated mode .
- Gravimetric method – method used for determination of sulfate ions content in concrete sample, basing on the reaction of sulfates with barium chloride in acidic solution; hardly soluble barium sulfate is precipitated and the content of vial is dried and weighed .
- Ion chromatography – adequate chromatography columns and eluents are used for analyses of water - soluble anions and cations in concrete. Concrete samples are pre-processed and extracted with distilled water.
Types
- Potentiometric titration – modern titrators are fully automated in data collection and calibration, all setup is made on the interface through software application to the equipment; usually titrators consist of with an Ag/ AgCl electrode with AgCl coating. Apparatus and equipment used in potentiometric titration for concrete consist of chloride-ion selective electrode, pH/mV meter and compatible automatic temperature compensation probe, adjustable-volume digital pipette, glassware: beakers, graduated cylinders, magnetic stirrer and teflon-coated stirring bars;
- Chloride Diffusion Test/ Ions migration in electrical field - testing apparatus consists of an upstream and downstream reservoirs and an electrical power source.
- Galvanostatic pulse technique - apparatus consists of battery and constant current source; stable current is applied - 1, 0.1 or 0.01 mA; A pulse duration of between 45 to 180 seconds can be used with a sampling rate of 1000 Hz to collect the dynamic response data.
- Argentometric titration – method can be generally divided into three commonly used types:
- Mohr method – yellow potassium chromate is used as an indicator in the titration of chloride ions with silver nitrate solution. In the beginning chloride ions will react with silver nitrate solution and form white precipitate of silver chloride. Endpoint is determined by chloride ions precipitation and if there is an excess - precipitation of red/brown silver chromate.
- Volhard method - excess amount of silver nitrate is be added to the sample solution to react with the chloride ions and form white precipitate of silver chloride; unreacted silver ions are then titrated with a standard solution of potassium thiocyanate in the presence of ferric ammonium sulfate solution (which acts as an indicator; the free silver ions had been completely reacted with thiocyanate ions, the first excess thiocyanate ion will react with the ferric ions of the indicator to form the red ferrithiocyanate complex, indicating the endpoint;
- Fajans method – in this method an adsorption indicator such as dichlorofluorescein which is a weakly acidic dye that exists in ionized form in the solution. Colloidal silver chloride precipitates tends to adsorb silver ions or chloride ions which in excess in the solution.
- Gravimetric method – in general there are three different types of gravimetric analysis:
- precipitation gravimetry – this type is applied for determination of sulfate ions in concrete sample; basing on the precipitation of the compound,
- volatilization gravimetry – basing on the thermal or chemical energy to remove a volatile compound,
- particulate gravimetry – basing on the separation of the analyte from the sample by filtration or extraction.
- Ion chromatography – typical modern chromatograph consists of single pump, conductivity detector, autosampler, eluent generator, cartridge, suppressor, analytical column, guard column; can be operated with various types of detection; sample processor processes samples from 500 µL to 500 ml; the sample transfer takes place by means of a peristaltic pump; There are two main types of ion chromatography:
- anion exchange chromatography,
- cation exchange chromatography.
Process/event to be detected or monitored
- Potentiometric titration - corrosion risk of rebars resulting from the influence of chlorides ions.
- Chloride Diffusion Test/ Ions migration in electrical field - chloride penetration level, capacity of concrete to resist chloride ions penetration.
- Galvanostatic pulse technique – detection of corrosion parameters of reinforcement ; dynamic response to a galvanostatic pulse.
- Argentometric titration – corrosion risk of rebars resulting from the influence of chlorides ions; observations of change of colour at the endpoint.
- Gravimetric method – expansion, cracking, strength loss and disintegraton of the concrete due to the presence of sulfate ions.
- Ion chromatography – detection and quantification of harmful ions and its influence on the structure integrity.
Physical quantity to be measured (e.g. actions, displacements, deformations, dynamic structural properties, material properties including mechanical, electrical and chemical properties, relative displacements of the two sides of a crack, etc.).
- Potentiometric titration - the potential or potential changes of the indicator electrode.
- Chloride Diffusion Test/ Ions migration in electrical field - concentration of the chloride ions in the sample.
- Galvanostatic pulse technique - corrosion current density measurement, polarization resistance change [mV] in potential of the steel reinforcement in time [S].
- Argentometric titration – chloride ions concentration in concrete sample.
- Gravimetric method – sulfate ions concentrations in concrete sample.
- Ion chromatography – concentrations of the ions present in the aqueous phase of concrete.
Induced damage to the structure during the measurement
For the mentioned experimental methods it is necessary to collect samples in forms of bore-holes or powdered samples from the inspected area, which induce damage to the structure in both cases.
General characteristics
Measurement type (static or dynamic, local or global, short-term or continuous, etc.)
- Potentiometric titration - static, local and short-term measurement.
- Chloride Diffusion Test/ Ions migration in electrical field - static, local and short-term measurement.
- Galvanostatic pulse technique - static, local and short-term evaluation.
- Argentometric titration - static, local and short-term evaluation.
- Gravimetric method - static, local and short-term evaluation.
- Ion chromatography - static, local and short-term evaluation.
Measurement range
- Potentiometric titration - balance sensitive to 0.0001 gram with a minimum capacity of 100 grams and volume digital pipette, with a range of 0.200 to 1.000 mL are usually used.
- Chloride Diffusion Test/ Ions migration in electrical field – range (×10-12 m2/s).
- Galvanostatic pulse technique - the applied current is usually in the range of 5 to 400 A and the typical pulse duration is 5 to 10 seconds.
- Argentometric titration - automated precipitation titrators work in measuring range: ± 2000 mV / 0–14 pH.
- Gravimetric method – not applicable.
- Ion chromatography – concentrations in range - µg/l (sub ppb) to mg/l (ppm), 1 mg/mL (1000 ppm).
Measurement accuracy
- Potentiometric titration - pH/mV meter, with accuracy of 0,1 mV.
- Chloride Diffusion Test/ Ions migration in electrical field – precision of the diffusion coefficient determination has been analysed In literature - .
- Galvanostatic pulse technique - potential is measured to an accuracy of ±5 mV with the Ag/AgCl electrode. The electrical resistance is estimated to be measured with an accuracy of ± 5 %.
- Argentometric titration - titration can achieve a relative error of 0.1–0.2%. The principal limitation to accuracy is the difference between the end point and the equivalence point.
- Gravimetric method – up to 0,01 g, depends on the balance used.
- Ion chromatography - flow rate: Programmable Analytical: 0,001 – 10,000 ml/min.
Background (evolution through the years)
- Potentiometric titration - first potentiometric titration was performed in 1893 with mercurous solution and potassium chloride, bromide, iodide. There were used a mercury electrode with a mercury/mercurous nitrate reference electrode. Wilhelm Bottger developed the tool of potentiometric titration at Ostwald's Institute. Potentiometric titration was used to examine the differences in titration between strong and weak acids .
- Galvanostatic pulse technique - introduced in 1988 to when problems of the interpretation of corrosion risk of reinforcement occurred, in situations where half-cell potential method is applied in wet or polymer-modified concrete and access of oxygen is limited.
- Gravimetric method - technique was used to determine the atomic mass of many elements in the periodic table .
- Ion chromatography - technique was introduced in 1850 study the adsorption of ammonium ions on soils. In 1947 the technique was used for separation of rare earth. Further the technique was extended to separate anions such as chlorides, fluorides, nitrates, and sulfates. Modern solutions offer improved sensitivity of detection and improvement in speed of analysis
Performance
General points of attention and requirements
Design criteria and requirements for the design of the survey
- Potentiometric titration – titrators are designed for laboratory use only, because there are requirements of the environment that should be fulfilled: ambient conditions, no powerful vibrations, no direct sunlight, no corrosive and explosive gas atmosphere, no magnetic fields; after configuration of the system there should be no leaks in any part of the titrator; usually 2,5 g powdered samples of concrete are used.
- Galvanostatic pulse technique - concrete slabs of dimension 1000 x 1000 x 150 mm can be used for testing.
- Argentometric titration - sample solutions have to be adjusted to near neutral pH, since at higher value, brown silver hydroxide precipitate may form and mask the endpoint.
- Ion chromatography – liquid samples have to be filtered out for removal of sediments or other impurities present in the sample.
Procedures for defining layout of the survey
Sensibility of measurements to environmental conditions
Preparation
Procedures for calibration, initialisation, and post-installation verification
According with the documents and standards listed in paragraph 1.10 Existing standards and to manufacturer guidance and manuals.
Procedures for estimating the component of measurement uncertainty resulting from calibration of the data acquisition system (calibration uncertainty)
Requirements for data acquisition depending on measured physical quantity (e.g. based on the variation rate)
Performance
Requirements and recommendations for maintenance during operation (in case of continuous maintenance)
Criteria for the successive surveying campaigns for updating the sensors. The campaigns include: (i) Georeferenced frame, i.e. the global location on the bridge; (ii) Alignment of sensor data, relative alignment of the data collected in a surveying; (iii) Multi-temporal registration to previous campaigns; and (iv) Diagnostics.
Reporting
In all mentioned methods modern software enable to automatically create reports after series or single measurement. Potentiometric titration software, for example AT- Win provide tools for automatic reporting to MS Word format. Titration test report usually contain the following elements: name and surname of the person carrying out the analysis, date of analysis, reactions, all obtained titration results, calculated mean mass of Cl- ions in the original sample for each method, compare the results obtained and any possible comment of discrepancies between them as well differences in their accuracy.
Example of the data report for Argentometric titration is shown below:
| Name and Surname: | Argentometric titration of chloride ions content | Date: | |||||
| Volume of the volumetric flask used [cm3] | |||||||
| Capacity of the measuring pipette used [cm3] | |||||||
| Commensurability of the flask with the pipette | |||||||
| Titre of the AgNO3 solution used [M]: | |||||||
| The titer of the used KSCN or NH4SCN solution [M] | |||||||
| MCl[g/mol] | |||||||
| Mohr Method | |||||||
| Indicators | |||||||
| Reactions | |||||||
| Lp. | V | [cm3] | Remarks | Average V | [cm3] | mass | [g] |
| 1. | |||||||
| 2. | |||||||
| 3. | |||||||
| 4. | |||||||
| Average content of chloride ions Cl- | |||||||
Lifespan of the technology and required maintenance (if applied for continuous monitoring)
Quantitative analytical methods are not used for continuous monitoring.
Interpretation and validation of results
Expected output (Format, e.g. numbers in a .txt file)
- Potentiometric titration – titration curves - plot of change of the potential vs volume of the added titrant reagent [mL].
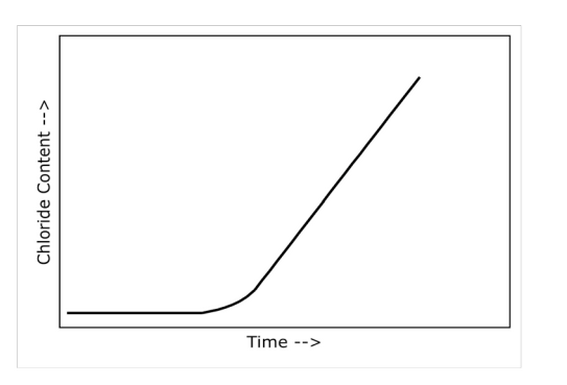
- Chloride Diffusion Test/ Ions migration in electrical field - plot of concentration changes of chlorides penetrating the cathode in time [s] – migration profile as shown below:
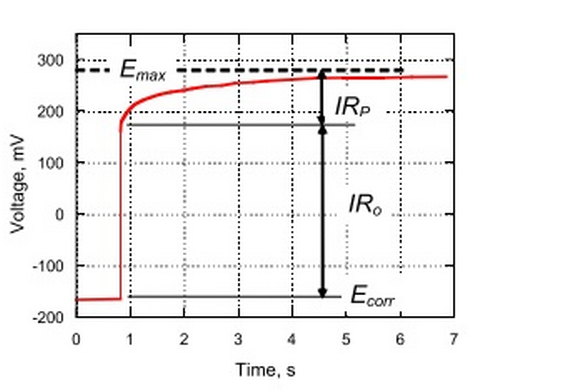
- Galvanostatic pulse technique – graph of the potential changes [mV] in time [s]; if used with 3D reporting software – 3D plot of the corrosion rates; below there is shown an example of the graph after application of the galvanostatic pulse:
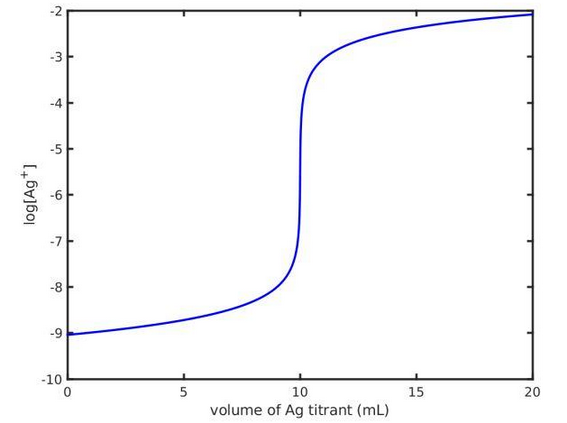
- Argentometric titration – titration curves - plot of logarithm of concentration of [Ag+] ions vs. volume of Ag titrant added [mL].
- Gravimetric method – list of percentage content by sample weight of sulfate ions, mean values.
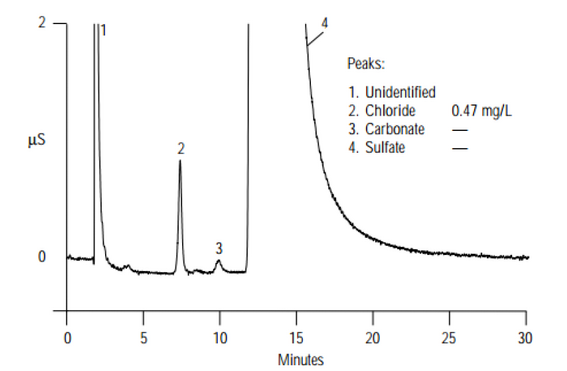
- Ion chromatography – the data is presented in a chromatogram - electrical conductivity versus time as the analyte passes through the system. Consists of several peaks corresponding to the different times in which components of the analyte emerge from the column, as shown below:
Interpretation (e.g. each number of the file symbolizes the acceleration of a degree of freedom in the bridge)
- Potentiometric titration - concentration of a given analyte based on titration curves.
- Chloride Diffusion Test/ Ions migration in electrical field - concentration changes of chlorides penetrating the cathode are used for determination of diffusion coefficient.
- Galvanostatic pulse technique – the corrosion rate is linked with the magnitude of corrosion current density; this value can be calculated using the charge transfer resistance from acquired data; charge transfer resistance is measured by summation of the individual resistances of components associated with corrosion.
- Argentometric titration - in Volhard method for example - the concentration of chloride ion in the sample solution is determined by subtracting the titration findings of the moles of silver ions that reacted with the thiocyanate ions from the total moles of silver nitrate solution that added.
- Gravimetric method – quantified sulphates concentration across the thickness of tested concrete elements; collected data indicate penetration length over the sample thickness and concentrations in the cross-section; determination of the damage depth of the tested elements due to sulfates aggression.
- Ion chromatography – concentrations of the ions of interests are calculated automatically and given by the instrument.
Validation
Specific methods used for validation of results depending on the technique
- Potentiometric titration results of the potentiometric titration should be valuated with another instrumental technique such as ion chromatography.
- Chloride Diffusion Test/ Ions migration in electrical field - linear, Freundlich, and Langmuir isotherms are used to fit the relationship between the experimental data;
- Galvanostatic pulse technique, Argentometric titration, Gravimetric method, Ion chromatography:
Quantification of the error
- Random errors: Random errors cause positive and negative deviations from the average value of a measurement. Random errors cancel by averaging, if the experiment is repeated many times. Upon averaging many trials, random errors have an effect only on the precision of a measurement. Solution is to increase the number of replicates performed to obtain a more trustworthy mean value.
- Systematic errors: Without any changes in the procedure, systematic errors are repeated if the experiment is repeated. Systematic errors have a biased effect on the final results. Systematic errors make the final result high or low, but not both. Instrument calibration errors are examples of systematic errors.
- Gross errors: Blend of systematic and random errors.
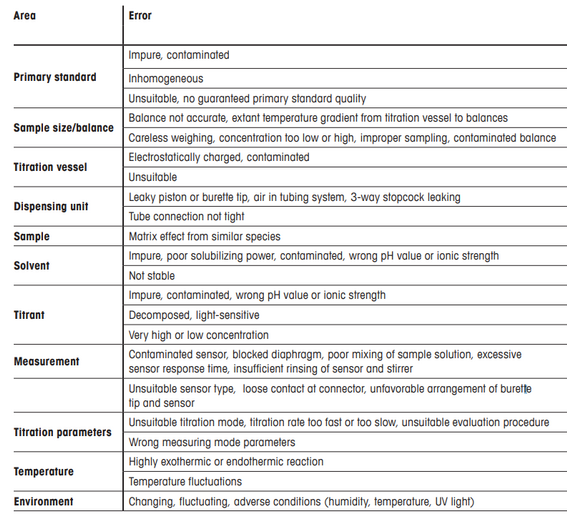
Sources of the errors in titration methods are listed below:
Quantitative or qualitative evaluation
Detection accuracy
With electrical resistance tomography it is possible to detect cracks with size below 1 mm.
In titration methods the values of repeatability and reproducibility can be determined following the standard ISO 5725-1981.
Advantages
- Potentiometric titration:
- can be performed even in the presence of a small amount of solute whose concentration is to be determined.
- Chloride Diffusion Test/ Ions migration in electrical field
- fast and effective method to determine the corrosion resistance of reinforced concrete against chloride.
- Galvanostatic pulse technique
- rapid evaluation.
- Argentometric titration
- very clear colour change at the end point of titration
- feasible to automate the process
- does not require specialized chemical knowledge.
- provides fast and precise result of the analysis
- Gravimetric method
- instrumental error is usually excluded
- does not require a series of standards for calculation.
- Ion chromatography
- accurate quantitative analysis
- identification and quantification of low concentrations of ions in the sample
- low maintenance costs and long-life of the apparatus used.
Disadvantages
- Potentiometric titration
- variation in electrolyte pH alters the result of titration
- electrolyte used in the reaction must be freshly prepared.
- Chloride Diffusion Test/ Ions migration in electrical field
- simple to operate.
- Galvanostatic pulse technique
- not possible to distinct between passive and actively corroding rebars.
- Argentometric titration
- requires practice to achieve effective results
- high background ionic level leads to errors.
- Gravimetric method:
- time consuming
- small mistake during the measurement may affect the final result.
- Ion chromatography
- only ions concentrations can be determined - complete salt-phases are determined by deduction
Possibility of automatising the measurements
Modern equipment for titration and chromatography is fully automatic in most cases, however the preparation stage of the samples and operations on the software cannot be omitted, which require presence of the qualified personnel.
Automating a titration analysis means more than simply having the titration and results calculation performed by automatic titrators. It must also include sample preparation steps and operator-independent sample series analysis. The main focus of automation is keeping actions throughout the process consistent to eliminate systematic, random, and even gross errors during routine tasks .
Barriers
Quantitative chemical analysis requires access to tested areas in order to collect representative samples from different structural elements. Analytical analysis is performed in the laboratory and thus requires additional time planned in advance if applied for periodic inspections.
Existing standards
- German Society for Non-destructive Testing Recommendations: Recommendations for Electrochemical Potential Measurement for Corrosion Detection in Reinforced Concrete Structures;
- AS 1012.20-1992, Method of Testing Concrete - Determination of chloride and sulfate in hardened concrete and concrete aggregates;
- PN-EN 14629:2008, Products and systems for the protection and repair of concrete structures -Test methods - Determination of chloride content in hardened concrete;
- PN-EN 1767:2008, Products and systems for the protection and repair of concrete structures - Test methods - Infrared analysis;
- ASTM C 1152, Test for nitric acid-soluble chloride content in concrete;
- ASTM C 1218, Test for water-soluble chloride content in concrete;
- ASTM D 4327, Standard Test Method for Anions in Water by Suppressed Ion Chromatography;
- PN-B-06714-46:1992 Kruszywa mineralne – Badania – Oznaczanie potencjalnej reaktywności alkalicznej metodą szybką;
- ISO 5725–94, Precision of test methods - Determination of repeatability and reproducibility for a standard test method by inter-laboratory tests;
- ASTM C 1202, Chloride ion migration-diffusion test.
Applicability
Relevant knowledge fields
- Civil engineering:
- corrosion diagnostics in concrete structures
- underground anthropogenic objects diagnostics.
- Geotechnics and Hydrotechnics:
- landlside risk assessment
- mapping of slope deformations
- slope stability monitoring
- river dike stability
- monitoring of processes in subsoil.
- Environmental studies:
- ground water penetration
- sources of contamination
- determination of water chemistries in aquatic ecosystems
- determination of chloride in sea water.
- Welfare and public health:
- monitoring of lead in water for consumption
- determination of sugar and salt content in foods
- analysis of cyanide, ammonia etc. in water or wastewater.
- Biology and medicine:
- water content in lyophilized vaccines
- 8 determination of plasma volume
- determination of the active pharmaceutical ingredients
- clinical diagnosis
- monitoring of ions in body tissue, blood
- analysis of metals
- drug analysis
- Material Engineering:
- nickel content in stainless steel
- corrosion diagnostics
- explosives analysis.
- Food Industry
- detection of perchlorate vegetables, milk
- detection of sugars in alcoholic and non-alcoholic drinks
- detection of acids in drinks and beverages
- Chemical Industry:
- detergents manufacturing
- detection of different elements in fertilizers
- paints analysis.
- Textile industry
- Oil and gas industry
- determination of acid number in crude oil.
Performance Indicators
- loss of section
- cracks
- obstruction/impeding
- displacement
- debondingspalling
Type of structure
- reinforced concrete slabs of the load-bearing bridge structure
- abutments of the bridge
- cable-concrete girders
- slabs
- concrete beams
Spatial scales addressed (whole structure vs specific asset elements)
Chemical analysis is addressed for components of the reinforced structures particularly subjected to the corrosion.
Materials
- concrete
- reinforced concrete
- ceramics
- polymer
- composites
- biomolecules
- nanomaterials
Available knowledge
Reference projects
- PARTNER (2002-2006) – founded by the European Community:
Objective – establishing a unified test procedure for evaluating the potential alkali – reactivity of aggregates across the different European economic and geological regions. Published by the Norwegian research institute SINTEF, Mannvit Engineering in Iceland, Verein Deutscher Zementwerke e.V in Germany, Associate - Building Research Establishment in England and SP Technical Research Institute of Sweden. * RILEM TC 219 ACS Alcali-Aggregate Reactions in Concrete Structures Committee.
Other
GAMRY INSTRUMENTS – Pulse Voltammetry Software
Bibliography
- Bertolini, L. (2013). Corrosion of steel in concrete : prevention, diagnosis, repair. Weinheim: Wiley-VCH.
- Butler, J. N. (1963, February). Calculating titration errors. J. Chem. Educ. 40, 2, 66, American Chemical Society and Division of Chemical Education, Inc.
- Climent, M. A., Viqueira, E., Vera, G. d., & López-Atalaya, M. M. (1999). Analysis of acid-soluble chloride in cement, mortar, and concrete by potentiometric titration without filtration steps. Cement and Concrete Research, vol. 29 no 6, pp. 893-898.
- D.W.Law, S. ,. (2001, January). The use of galvanostatic pulse measurements to determine corrosion parameters. British Corrosion Journal, pp. 1-10.
- E Poulsen, L. M. (2014). Diffusion of Chloride in Concrete: Theory and Application. . Boca Raton: FL : CRC Press.
- Gerardo G. Clemeña, C. M. (2002). An Alternative Potentiometric Method for Determining Chloride Content in Concrete Samples from Reinforced-Concrete bridges. Charlottesville, Virginia: Virginia Transportation Research Council.
- James A. Farny, B. K. (2007, July 21). Diagnosis and Control of Alkali-Aggregate Reactions in Concrete. Portland Cement Association, pp. 1-26.
- Jóźwiak, H. (2009). Możliwości wykorzystania metody jądrowego rezonansu magnetycznego w badaniach domieszek chemicznych w betonie. Prace Instytutu Techniki Budowlanej, pp. 3-13.
- K.D. Stanish, R. H. (1997). Testing the Chloride Penetration Resistance of Concrete: A Literature Review. Toronto, Ontario: University of Toronto.
- L. Tang, H. E. (2001, 10). Precision of the Nordic test methods for measuring the chloride diffusion/migration coefficients of concrete. Materials and Structures/Matériaux et Constructions, Vol. 34, pp. 479-485.
- Mettler Toledo International Inc. . (2015). Titraion Guide . Greifensee: Mettler Toledo .
- Nadejda V. Orlova, J. C. (1999). The study of chloride ion migration in reinforced concrete under cathodic protection - Final Report (SPR 357). Oregon : Oregon Department of Transportation.
- Nordtest. (1995, November 22). Concrete, hardened: Accelerated chloride penetration. Retrieved from http://nordtest.info/images/documents/nt-methods/building/NT%20build%20443_Concrete,%20hardened_Accelerated%20chloride%20penetration_Nordtest%20Method.pdf
- R. Vedalakshmi, L. B.-H. (2010, March 3). Reliability of Galvanostatic Pulse Technique in Assessing the Corrosion Rate of Rebar in Concrete Structures: Laboratory vs Field Studies. KSCE Journal of Civil Engineering , pp. 867-877.
- S. Sathiyanarayanan, P. N. (2006, May 24). Corrosion monitoring of steel in concrete by galvanostatic pulse technique. Cement & Concrete Composites, pp. 630-637.
- Shing, G. Y. (2014). A comparision of three methods used for determining cholride in acid copper sulfate plating bath. Kuala Lumpur: Faculty of Science University of Malaya .
- Stark, D. (1991). Handbook For The Identification Of Alkali Iica Reactivity In Highway Structures. Washington: Strategic Highway Research Program National Research Council.
- Thomas Frølund, F. M. (2002). Smart Structures: Determination of reinforcement corrosion rate by means of the galvanostatic pulse technique. First International Conference on Bridge Maintenance, Safety and Management (pp. 1-9). Barcelona: IABMAS.
- U.S. Dept. of the Interior, B. o. (1992). Concrete manual. U.S. Dept. of the Interior, Bureau of Reclamation.
- Zofia Szweda, A. Z. (2012). Wyznaczanie współczynnika dyfuzji chlorków w betonie na podstawie badań migracji jonów w polu elektrycznym. Trwałość Budowli i Ochrona przed Korozją, pp. 60-62.